📢 Highlights
Owkin, Bioptimus Develop Smaller, Faster AI Model for Digital Pathology
Illumina Announces Supply Chain Challenges Amidst President Trump’s Trade War
Following Failed Partnership, Affibody Takes Back Control of Key Asset from Acelyrin
Not yet a member of our super awesome slack community of >8500? Join HERE 🤗
👀 In Case You Missed it ..
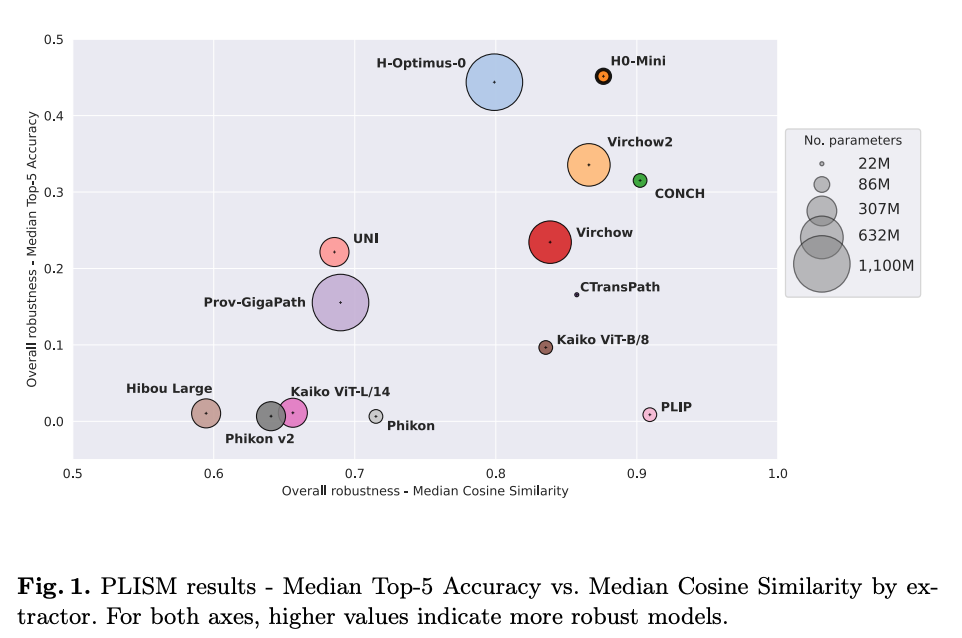
Distilling Foundation Models for Robust and Efficient Models in Digital Pathology
Research on foundation models for digital pathology is growing for tasks such as biomarker prediction, gene expression analysis, and tissue classification. This new paper by Owkin and Bioptimus teams explores distilling large foundation models to create smaller, more efficient models without sacrificing performance. The authors distilled H-Optimus-0, a model with over 1 billion parameters, into H0-mini, a compact version with 86 million parameters. Despite its reduced size, H0-mini achieved competitive results on benchmarks like HEST and EVA and demonstrated superior robustness to variations in tissue staining and scanning on the PLISM dataset. Thanks to its efficiency and robustness, H0-mini is a strong contender for clinical use, with lower computational costs without compromising performance or reliability in real-world settings.
Illumina Faces Challenges Amid U.S.-China Trade Tensions and Tariffs.
Finding fellowship amongst myriad industry players whose operations and strategies have been upended by recent regime changes in the White House, the legendary genetic sequencing company Illumina has reported it has been significantly impacted by recent U.S.-China trade tensions, resulting in increased tariffs on its products. The company is navigating these challenges as it continues to provide sequencing technologies globally, while admitting the possibility of distributions, delays, and price changes. The broader implications of these trade dynamics on the biotech industry remain to be seen. Illumina is assessing the potential impact on its supply chain and exploring strategies to mitigate the effects of the tariffs. The situation underscores the complexities global companies face amid shifting international trade policies amongst all industries, including those whose products and services influence life and death decisions on a daily basis.
Behind the Deal: Affibody’s Second Act - Seizing Opportunity with Izokibep
Affibody recently announced that it has regained the rights to izokibep, an autoimmune drug candidate it once licensed to Acelyrin. The partnership originally sparked high expectations: Acelyrin paid $25 million upfront in 2021, secured $540 million in IPO funding, and pursued izokibep as a potential breakthrough for conditions like hidradenitis suppurativa, a chronic inflammatory skin condition. However, despite meeting primary endpoints in phase 3 trials, Acelyrin halted its investment, citing challenges in capitalizing on the drug’s potential. Acelyrin’s CEO, Mina Kim, suggested the asset required resources and market reach beyond the company’s capacity. This decision illustrates a critical lesson for investors: even promising drugs can falter if the necessary commercial infrastructure or strategic focus is lacking. With recent clinical setbacks in other indications, Acelyrin ultimately chose to return the rights. Now, Affibody sees an opportunity. CEO David Bejker remains optimistic, positioning izokibep as a game-changer for dermatological conditions. With a solid phase 3 data package, Affibody intends to tap into the drug’s potential where Acelyrin left off. By reclaiming izokibep, Affibody signals its intention to reassert itself in the autoimmune space. Whether through new partnerships or an independent path, Affibody’s approach will be closely watched as an indicator of how smaller biotech companies can navigate complex development and commercialization challenges.
Recursion Expands AI Application into Clinical Trials
Utah-based Recursion Pharmaceuticals is advancing its AI capabilities beyond drug discovery into clinical trials. Known for its automated and high throughput morphology-based screening platform and the petabytes of data it has produced, the company is turning its attention downstream in the developmental process - with at least seven programs anticipated to enter human trials or yield clinical data in 2025, the company has initiated a "ClinTech" effort. This initiative aims to enhance trial design, expedite patient enrollment, and improve evidence generation. By leveraging AI, Recursion hopes to optimize study protocols, identify suitable patient populations more efficiently, and generate robust clinical evidence, thereby accelerating the development timeline and increasing the likelihood of successful outcomes, “First, it’s really around smarter trial design programs. Second, it’s around accelerating enrollment. And the third is around enhancing evidence generation,” Najat Khan, PhD, Recursion’s chief R&D officer and chief commercial officer.
Molecular-driven Foundation Model for Oncologic Pathology
Researchers from Harvard Medical School, Massachusetts General Hospital, and the Broad Institute have developed THREADS, a slide-level foundation model that integrates histology images with genomic and transcriptomic data to generate comprehensive whole-slide embeddings. THREADS is trained on over 47,000 tissue sections from 39 organs and captures tissue morphology and molecular profiles. It outperforms models like PRISM and GIGAPATH across 54 oncology tasks, including mutation prediction, treatment response, and survival analysis. THREADS performs well in data-scarce scenarios, offers robust off-the-shelf embeddings for clinical tasks, and supports fine-tuning, making it a versatile tool for computational pathology. In addition to the preprint, a longer form description is available through the authors’ team’s blog post.
8VC Targets $1 Billion Fund to Invest in Tech and Life Sciences
The well known Bay-area venture capital firm 8VC, known for backing companies like Altos Labs, is aiming to raise a $1 billion fund focused on technology and life sciences investments. The firm’s 6th funct, the focus of which reflects the firm's commitment to supporting innovative startups at the intersection of technology and biology. The fund is expected to fuel advancements in areas such as biotechnology, healthcare, and computational biology. 8VC's strategy involves identifying and nurturing early-stage companies that leverage technological innovations to address complex challenges in life sciences. The raise is likely to be successful given the VC’s founder, Joe Lonsdale, has built his career on co-founding the defense IT contracting firm Palantir.
Astellas Announces Management Restructuring Amid Strong Financial Performance
Japan-based biopharmaceutical powerhouse, Astellas Pharma, has initiated a major management restructuring, effective April 1, 2025. To call it a bloodbath may not be inappropriate. Included amongst the sudden seniormost departures: General Counsel Catherine Levitt, chief manufacturing officer Hideki Shima, and Chief Scientific Officer Yoshitsugu Shitaka, Ph.D. Additionally, Chief Medical Officer Tadaaki Taniguchi, M.D., Ph.D., will be moved to a newly created position of chief research and development officer. This move is being reported as a means of streamline operations, increasing efficiency, and enhancing decision-making processes. Normally such changes occur on the backfoot of significant corporate decline, but in this case it is the opposite - Astellas reported a robust nine-month earnings, driven by the expansion of sales of XTANDI™ (enzalutamide) for the treatment of prostate cancer that was approved in 2023.
Multi-Modal Transformer Enhances Cell Type-Agnostic Regulatory Predictions
A recent study published in Cell Genomics introduces a multi-modal transformer model designed to improve regulatory genome predictions across various cell types. This model integrates multiple data modalities, including genomic sequences and epigenetic information, to predict regulatory elements without being confined to specific cell types. The approach enhances the generalizability and accuracy of regulatory predictions, facilitating a deeper understanding of gene regulation mechanisms across diverse biological contexts. This advancement holds potential for broad applications in genomics and personalized medicine, but its impact will take time to manifest and hard to index to this particular innovation.
AdvanCell Secures $112 Million in Series C Funding For Radiopharmaceutical Therapies
The Australian radiopharmaceutical company AdvanCell has completed a $112 million Series C. The funding, led by Sanofi Ventures, will support the progression of AdvanCell's lead asset, a targeted alpha-emitting radiotherapy, into clinical trials. This investment represents a significant step in AdvanCell's journey, which started in relative obscurity in 2019, to become a global leader in radionuclide-based cancer therapeutics. Their approach focuses on leveraging alpha-emitting isotopes to deliver targeted radiation therapy, improving efficacy while minimizing damage to surrounding healthy tissues. The company plans to utilize the funds to advance its pipeline and expand its manufacturing capabilities beyond its 40,000 core operating site to meet anticipated demand.
Owkin Administers First Dose of Its AI-Optimized Cancer Therapy
The French Techbio titan, Owkin, has commenced its INVOKE clinical trial by dosing the first patient with OKN4395, a first-in-class EP2/EP4/DP1 triple inhibitor designed to counteract cancer's immune evasion mechanisms. Developed using Owkin's AI-powered K1.0 operating system, OKN4395 targets prostaglandin receptors that tumors exploit to suppress immune responses. The Phase I study will assess the drug's safety, tolerability, and preliminary anti-tumor activity, both as a monotherapy and in combination with pembrolizumab. This trial marks a significant milestone in applying AI to accelerate the development of novel cancer therapies.
Lantern Pharma Launches AI-Driven Platform for Antibody-Drug Conjugate Development
The Dallas Texas based, and publicly traded, company Lantern Pharma has announced advancements in its RADR platform, integrating a new AI-powered module designed to accelerate and optimize the development of antibody-drug conjugates (ADCs). The enhanced platform has allegedly identified 82 potential ADC targets, including 22 that are clinically validated, along with 60 novel targets with potential for intellectual property and portfolio expansion. The company additional stated that RADR has screened over 50,000 compounds, validating 729 payload molecules with therapeutic potential. Lantern's approach leverages AI to analyze complex genomic and transcriptomic datasets, enabling the identification of optimal target-payload combinations for ADCs. The company estimates that this AI-driven approach could reduce ADC development timelines by 30–50% and lower costs by up to 60% compared to traditional methods.
🗓️ Upcoming BiB Events
See our handy dandy Lu.ma event calendar HERE, please RSVP so folks can plan accordingly!
Bits in Bio Philadelphia February Happy Hour, Thursday Feb 6th at 5PM, at Landmark Americana University City (yes, it is a bar)
Thank you to AJ Adejare for organizing!
Coffee Connections in Somerville Mass., Saturday Feb 9th at 10AM, at Remnant Brewing - Taproom, Cafe, and Smiles.
Thank you to Traci Haddock and Nelly Tian for organizing!
📰 Top Community Conversations
Nothing to highlight this week - check out the BitsInBio Q&A channel to get your fix!
🏢 New Job Openings
Notable postings below - over 100 more on our community job board!
Graduate Research Fellow - Genomics Scientist (Summer 2025) at Axiom Labs
Junior Data Scientist at Polymodels Hub
Part-Time Coding Project at OpenAI
Postdoctoral Researcher, Open Datasets Initiative at Align to Innovate
Principal Software Developer at OXB
Senior Director/Director, Software Product at Lila Sciences
🙋 🙏 Community Asks
Feedback: How is the Newsletter doing? We’re trying different formats/content. In case the hyperlink above didn’t get your attention, maybe a bright orange button will!
Volunteer: Want to get involved with Bits in Bio, meet new members across the community, and learn about the ecosystem? We are looking for volunteers to help us create great content and manage the community.
🙏 Thank you for being a BiB Weekly reader!
We want to deliver what matters most in Bio AI and would love your feedback on how we can do better. Please weigh in as anon here or DM me directly!