📢 Highlights
NIH’s New ORIVA Office Launched to Support Human-First Research
Four Literature-Crunching AI Scientist Agents Take Flight at FutureHouse
Coinbase Cash Backs $130M Series B in Brian Armstrongs Epigenetic Fountain of Youth - NewLimit
Not yet a member of our super awesome slack community of >9000? Join HERE 🤗
👀 In Case You Missed it …
NIH Launched New Office, ORIVA, Betting Its Money on Human-First Research
On the footsteps of its recent announcement of curtailing the use of animal models in the approval process for new biologics, the NIH is launching a proactive new initiative to prioritize human-based research models (think organoids and “tissue chips”) and reduce reliance on animal testing. The agency will set up a new office (ORIVA) to fund and scale these non-animal approaches across its institutes, aligning with the FDA’s parallel push to cut back on animal tests. Emerging tech like organ-on-chip systems, AI-driven simulations, and real-world data will be tapped to tackle questions that animal studies often fail to translate to humans. NIH Director Dr. Jay Bhattacharya calls it “ushering in a new era of innovation” focused on human biology and faster breakthroughs . Importantly, NIH will retrain grant reviewers to curb any bias for animal studies and will publicly track its funding shift – concrete steps to ensure this human-first approach actually takes hold.
FutureHouse releases AI Agents for Scientific Research
FutureHouse just launched a platform giving access to a suite of AI agents designed to help researchers navigate scientific literature and data. Each agent has a clear focus: Crow for fast, accurate literature search, Falcon for deep reviews using sources like OpenTargets, Owl for checking research novelty, and Phoenix, still experimental, for planning chemistry experiments. The agents have been benchmarked to outperform existing models in retrieval precision and accuracy, surpassing PhD-level researchers in some of the search tasks. The platform is directly available through this link.
Behind the Deal: From Coinbase to Cellbase – Brian Armstrong’s NewLimit Raises $130M to Reverse Aging
NewLimit, the biotech venture co-founded by Coinbase CEO Brian Armstrong, has raised $130 million in a Series B led by Kleiner Perkins, boosting its valuation to $810 million. The company is developing epigenetic reprogramming therapies to restore youthful function to aged cells, with an initial focus on the liver.
NewLimit leverages artificial intelligence, single-cell genomics, and high-throughput functional assays to identify transcription factors that can rejuvenate old cells. Lab experiments have produced three prototype medicines that reprogram liver cells, restoring their ability to process fat and alcohol-key for tackling age-related liver diseases. However, all programs remain preclinical, and human trials are still several years away. The company acknowledges the long road ahead, emphasizing its 20-year ambition to significantly extend human healthspan. NewLimit joins a new wave of high-profile longevity startups, including Jeff Bezos-backed Altos Labs and Sam Altman-supported Retro Biosciences, both of which are also pursuing age-reversal breakthroughs. As Armstrong’s team sets its sights on extending human healthspan, this funding round signals growing momentum-and competition-in the race to turn age reversal from science fiction into clinical reality.
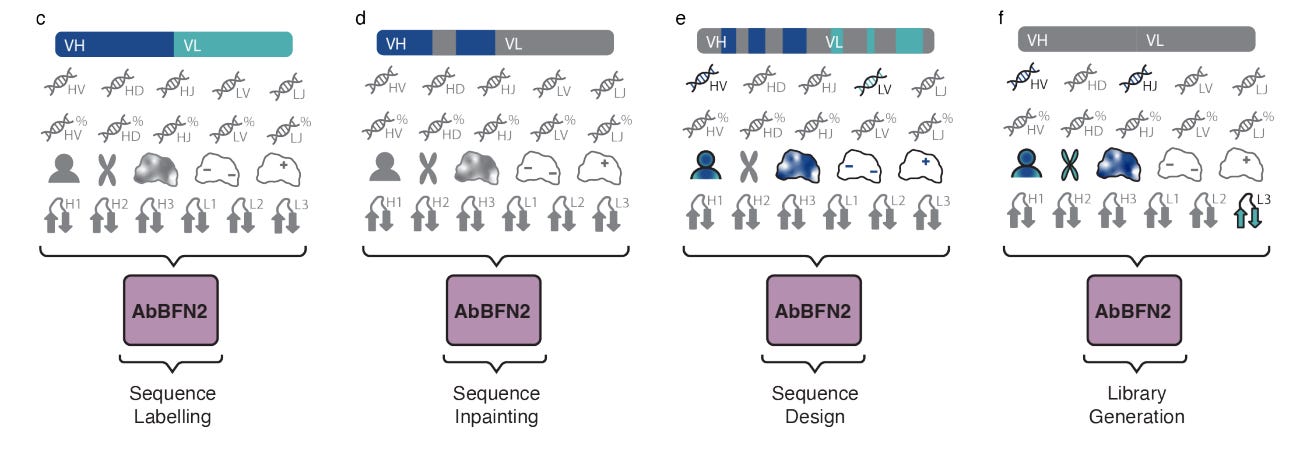
AbBFN2: A flexible antibody foundation model based on Bayesian Flow Network
Instadeep published AbBFN2, a generative foundation model for antibodies built on the Bayesian Flow Network (BFN) framework. It handles 45+ sequence, genetic, and biophysical features without task-specific training, unifying humanisation, inpainting, property optimisation, and de novo design in one model. Trained on 2M+ annotated antibodies, it learns natural antibody distributions and outperforms some other annotation tools. The model aims to enable multi-objective design in a single step and to target rare antibody types in order to offer a flexible platform for therapeutic antibody generation. The model can be found on the company’s github page.
Recursion tidies up Exscientia pipeline post-merger
It has been six months now since Recursion acquired UK-based Exscientia, the Salt Lake City based AI biotech is pruning its drug pipeline to refocus on cancer and rare disease R&D. The company is dropping three clinical programs and pausing another to double down on areas with stronger data, effectively Marie Kondo-ing anything that doesn’t spark joy (or results). This post-merger purge leaves Recursion with six active programs (four in oncology, two in rare diseases) that leadership says align with its “high-impact opportunities” strategy. On the upside, Recursion snagged a quick $7M milestone from an Exscientia-originated partnership with Sanofi – a hint that the acquisition is already paying dividends as the company streamlines. Overall, the message is clear: less pipeline clutter, more focus on what works (and maybe a little extra cash to boot).
Wiley & AWS Launch AI literature agent to Support Researchers
Publisher Wiley has teamed up with AWS to launch a generative AI agent that does full-text literature searches across Wiley’s journal content. Unveiled at AWS’s Life Sciences Symposium in New York, it’s the first AI “agent” from a major publisher to live on AWS’s platform. The agent comes via an open-source AWS toolkit for life-science AI agents, meaning researchers (or anyone) can use and tweak it for deep dives beyond the abstract – it scours methods, results, and all. By searching entire articles instead of just summaries, the tool aims to shrink literature review times from days to minutes. Wiley and AWS are pitching it as a way to embed trusted scientific content into research workflows, hinting that digging up insights from full-text data could help accelerate discoveries.
Twist Spin Out for DNA-based Data Storage Gets $155M Liftoff
Synthetic biology player Twist Bioscience just spun out its DNA digital storage unit into a new company, Atlas Data Storage, with a hefty $155 million in “seed” funding. Big-name backers like ARCH Venture, Deerfield, Bezos Expeditions, Tao Capital, and even In-Q-Tel (the CIA’s VC arm) are funding Atlas to commercialize DNA as a data storage medium. Twist will retain a minority stake in Atlas and license out its DNA storage technology, while refocusing on its core biotech business and aiming for profitability by 2026. Atlas, led by storage industry veteran Varun Mehta, will run with Twist’s tech via early access programs, pitching synthetic DNA as an ultra-dense, stable storage solution for the long-term archiving market. It’s a bold bet that DNA “hard drives” could one day sit alongside magnetic tape and cloud servers for data archiving – and with $155M behind it, that futuristic vision just got a big push.
Novartis Invests in microRNA Revival in Purchase of Regulus Therapeutics
Pharma giant Novartis is diving into the microRNA therapeutics game by acquiring Regulus Therapeutics, a long-struggling pioneer in RNA-based drugs. The pharma giant will pay $7.00 per share in cash (around $800 M upfront) plus another $7.00 per share contingent on a future milestone – roughly a $1.1 billion payout upfront with the total deal valued up to $1.7 billion. Regulus made its name developing oligonucleotide drugs targeting microRNAs, though the field had fallen out of vogue in recent years after some setbacks. Novartis’s interest suggests a renewed big-pharma faith in this approach, perhaps betting that Regulus’s lead candidate (a microRNA-targeting therapy called farabursen) can finally validate the concept. It’s a notable exit for a small company founded in 2007, and a signal that nucleic-acid medicines (like microRNA and siRNA therapies) remain hot targets for M&A.
Radium’s AI “Co-Pilot” for Tumor Segmentation Released
Paris-based startup Raidium has published a 3D tumor segmentation foundation model (dubbed ONCOPILOT) that radiologists can interact with using simple visual prompts. Trained on 8,000+ CT scans, the model lets a doctor draw a quick bounding box or click a point, and then it automatically outlines the tumor volume for fine-tuning. It’s a step up from old-school one-dimensional tumor measurements (RECIST), enabling full volumetric analysis and richer radiomic data from scans. Raidium – which raised €16M in late 2024 – frames ONCOPILOT as an “AI co-pilot” to boost radiologist efficiency and precision, not replace them, especially as imaging workloads grow. With the model now validated in npj Precision Oncology, the company is expanding into clinical research partnerships and prepping for regulatory approvals in the EU and U.S.
Recursion Trial Shows 43% Polyp Drop in AI-designed FAP Treatment
Salt Lake City based Recursion has shared positive early results from a Phase 1b/2 trial of REC-4881, a MEK1/2 inhibitor discovered via its AI platform, in patients with familial adenomatous polyposis (FAP). After 13 weeks on the daily pill, patients showed a median 43% drop in their intestinal polyp burden. Five of six patients had at least one-third of their polyps shrink (with some seeing >80% reductions), though one patient experienced a big increase in polyps despite treatment. Notably, half the treated patients improved by one Spigelman stage (a score of duodenal polyp severity), an encouraging sign in this cancer-prone condition that currently has no approved drug therapies. Side effects like rash and diarrhea were in line with other MEK inhibitors and mostly mild, so the trial is forging ahead, with more data expected in late 2025.
Lantern’s AI Drug Faces Triple-negative Breast Cancer
Texas-based Lantern Pharma just got FDA clearance to kick off a Phase 1b/2 trial of LP-184 in triple-negative breast cancer (TNBC) – a novel small molecule identified and developed using the company’s AI platform (RADR). The trial will evaluate LP-184 both as a standalone therapy and in combination with the PARP inhibitor olaparib for patients with recurrent TNBC. Notably, LP-184 already scored Orphan Drug status in 2023 and Fast Track status in 2024 for TNBC, underscoring the dire need for new options against this aggressive cancer. Lantern’s AI platform reportedly sifted through 100+ billion data points to pinpoint LP-184, exemplifying how big-data pipelines can accelerate the repurposing or design of niche oncology drugs. With the IND now green-lit, Lantern’s data-driven drug is heading into the clinic to test safety and early efficacy – aiming to improve on the bleak ~18-month survival for metastatic TNBC.
Lambic Offers AI Crystal Ball for Drug R&D - Enchant v2
Drug discovery startup Iambic unveiled Enchant v2, an AI model to predict whether early-stage drug programs will ultimately thrive or flop in the clinic. Enchant v2 is a transformer trained on a firehose of chemical, preclinical, and clinical data, boasting a >10× increase in model size over its predecessor. It outperformed both the original Enchant and Google’s new Gemma model on key pharmacokinetic and ADME benchmarks, showing it can wring insights from surprisingly little data. The goal is to let drug developers kill dead-end projects early and double down on promising ones, using AI to guide multi-parameter optimization and even clinical trial design. It’s a bold attempt to algorithmically foresee a drug program’s fate — if Enchant v2 lives up to the hype, it could shift how R&D portfolios are managed.
Generative cell atlas spans 1.5 billion years
Stanford-led researchers have built a gargantuan single-cell AI model that spans 1.5 billion years of evolution. Dubbed TranscriptFormer, this generative foundation model was trained on 112 million cells from 12 species, learning to represent gene expression across the tree of life. In zero-shot tests, it can recognize cell types even in species separated by ~685 million years, and even flag disease states in human cells without explicit training. Because it’s generative, you can prompt it to suggest which transcription factors drive a given cell type or how genes interact, and its answers align with known experimental results. The work hints at a future “virtual cell atlas” for doing cross-species experiments in silico – essentially an AI microscope to study evolution and cell biology from yeast to humans.
Vertex abandons AAV gene therapy delivery
For gene therapy watchers, it’s becoming clear that AAV viral vectors are losing favor. Vertex Pharmaceuticals just decided to scrap all its AAV-based delivery research, joining a growing list of companies pulling back from the once-dominant gene therapy method. The move doesn’t mean Vertex is done with gene therapy altogether – they’re still pushing their CRISPR-based sickle cell cure (just approved as Casgevy) and other cell and genetic treatments. But it echoes others: Takeda shelved its AAV programs, Pfizer yanked an AAV-based hemophilia therapy, and even Roche’s Spark unit is reorganizing away from AAV work. High costs, safety setbacks, and the lure of newer delivery tech (like gene editing or LNPs) are driving an industry rethink of AAV, marking a significant strategic pivot in the field.
Diffusion Model Attackes Peptide–MHC Puzzle to Predict T-cell Targets
Academic researchers have unveiled MHC-Diff, an AI diffusion model that predicts how peptides dock into MHC proteins – a crucial step for immune recognition. Unlike traditional docking simulations, MHC-Diff leverages geometric deep learning (a SE(3)-equivariant model) to generate peptide–MHC binding structures with near-atomic accuracy. It’s not just accurate, either – this generative approach can rapidly produce an ensemble of plausible peptide–MHC conformations, offering results in a fraction of the time of physics-based methods. Such speed and precision could turbocharge personalized cancer vaccine development, where scientists need to quickly identify which neoantigen peptides actually fit a patient’s HLA molecules. It’s yet another example of generative AI breaking new ground in biotech, hinting that even immunology’s trickiest shape-fitting problems might be tackled with clever neural networks.
AstraZeneca Bets on Data-driven Drug Startup Pathos
Meet Pathos AI: a stealthy Chicago biotech founded by former Tempus executives that’s blending real-world patient data and lab biology to revamp drug R&D. Pathos has quietly raised $283 million of a planned $400 million round, with AstraZeneca stepping in as a strategic investor. The startup leverages Tempus-style multimodal data (genomics, clinical records, etc.) plus patient-derived biological models to pinpoint new precision therapies. This massive war chest and Big Pharma backing suggest high ambitions to use AI at scale in drug development, likely focusing on oncology (as hinted by AZ’s involvement). Given the Tempus pedigree and the size of the raise, Pathos AI is definitely one to watch in the techbio arena.
Bay Area Live-cell Imaging Startup Exits Stealth with $12M
Led by a former Google machine learning engineer, Stately Bio has emerged from stealth with a $12 million seed round to advance its AI-powered live-cell imaging platform. The Palo Alto startup uses label-free, time-lapse microscopy plus custom ML algorithms to monitor living cells in real time. The tech can spot subtle changes in how cells grow and differentiate – without any fluorescent tags or high-end hardware – essentially giving a standard microscope an AI “brain”. The goal is to boost cell therapy and stem cell R&D by catching quality issues or optimal cell states early (“seeing” which cell colonies are the winners) in a way that human eyes or traditional assays might miss. It’s yet another example of Big Tech talent crossing into biotech, with VCs like AIX Ventures backing a new approach to make cell therapy development faster and more data-driven.
Research Study Shows HIV fusion Inhibitor Designed Entirely by AI
Using an AI-based protein design tool called EvoBind, scientists have created a novel cyclic peptide that blocks HIV-1 from fusing with host cells. In a “single-shot” design process (no iterative optimization needed), the team generated this peptide computationally using just the virus’s sequence and AlphaFold models of the target – no experimentally solved structure required. The resulting molecule showed low-micromolar inhibitory activity against HIV’s membrane fusion, with no detectable toxicity in initial tests (an impressive feat, given HIV’s rapid mutational escape). It’s a striking proof-of-concept for AI-driven drug discovery: even against a fast-changing target like HIV, a viable inhibitor can be virtually designed in one go. The work points toward a future where researchers could swiftly craft antivirals and other therapeutics entirely in silico, potentially shaving years off the drug development process.
🗓️ Upcoming BiB Events
See our handy dandy Lu.ma event calendar HERE, please RSVP so folks can plan accordingly!
SynBioBeta BiB Happy Hour with Trinet & Escalon Services , Wednesday, May 7
5:00 PM @ Mosaic Restaurant in San Jose, Ca
DataDrivenPharma Evening Forum at Bakar Labs, Wednesday, May 14
5:00 PM @ Woo Hon Fai Hall in Berkely
Chips & Cells: Advancing discoveries in cell membrane biology through AI & physics-based computational methods Friday May 16th in Berlin
Chicago Bits in Bio Happy Hour! Thursday, May 29 at Recess in Chicago
📰 Top Community Conversations
Check out some excellent resources from the community on lab automation
🏢 New Job Openings
Senior Product Designer at Aclid
Various Roles at QbDVision
Engineers at Apheris
Strategic Advisor, Clinical Pharmacology & PK/PD Modeling at Varosync
Computational Scientist II - Vallabh/Minikel Lab at Broad Institute
Engineers at Sobek AI
🙋 🙏 Community Asks
Feedback: How is the Newsletter doing? We’re trying different formats/content. In case the hyperlink above didn’t get your attention, maybe a bright orange button will!
Volunteer: Want to get involved with Bits in Bio, meet new members across the community, and learn about the ecosystem? We are looking for volunteers to help us create great content and manage the community.
🙏 Thank you for being a BiB Weekly reader!
We want to deliver what matters most in Bio AI and would love your feedback on how we can do better. Please weigh in as anon here or DM me directly!